Rare Perspectives: Factor X Deficiency
March 31, 2020Keys To Clinical Trial Success In Rare Disease Indications
This Atlantic Research Group rare disease case study is available for download as a PDF.
Introduction
Factor X (FX) deficiency is a rare bleeding disorder where affected individuals are not able to produce normal amounts of FX, a crucial enzyme during blood coagulation that helps form stable blood clots following blood vessel damage in the body.
Individuals with FX deficiency can experience mild to severe blood coagulation complications1.
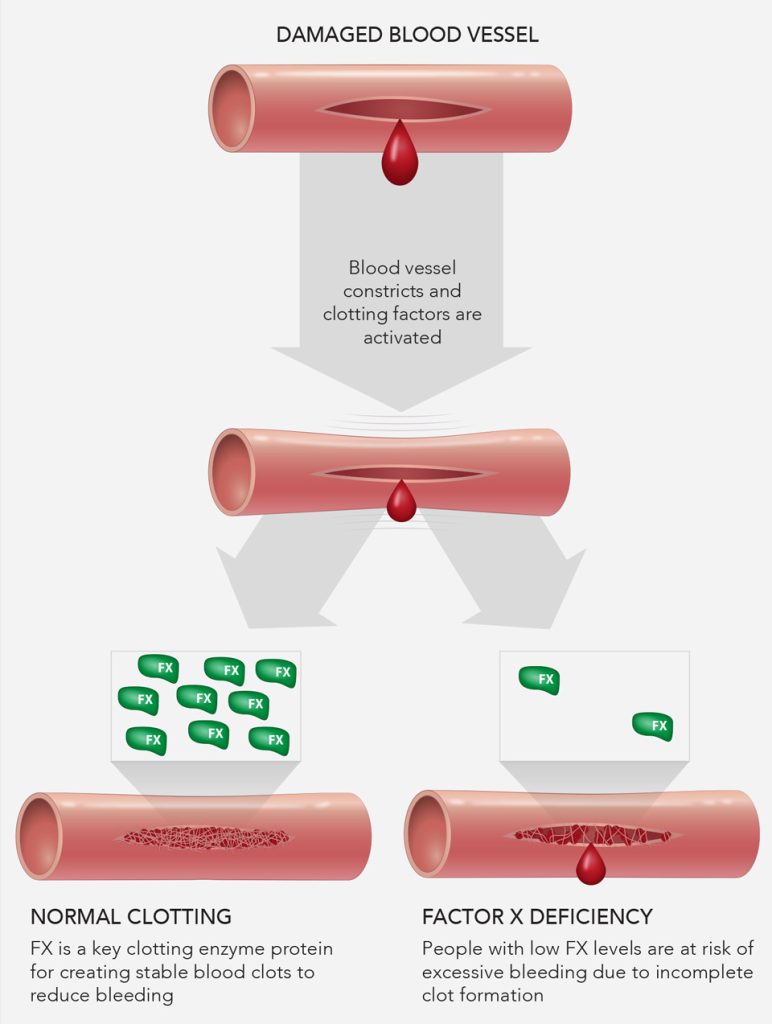
To date, there is only one FDA-approved treatment for hereditary FX deficiency on the market. However, planning for a successful clinical trial for FX deficiency requires more than disease awareness or in-depth knowledge of the underlying pathologies. In the realm of rare disease clinical research, there are additional considerations for therapeutic developers to contemplate. Some key questions sponsors may want to ask include, but are not limited to, the following: Does my study account for the variable symptoms/phenotypes seen among FX-deficient patients?
- Are study investigators accurately measuring FX levels in patients?
- Are sites that could potentially be treating FX-deficient patients properly identified?
- Is the trial intelligently utilizing hematologists and other KOLs
- Are all trial stakeholders leveraging the Hemophilia Treatment Centers (HTC) model to its fullest potential?
Based on its involvement in FX deficiency studies over the past decade, Atlantic Research Group (ARG) has developed a set of key concepts for sponsors to consider when engaging FX-deficient patients and clinicians, while keeping a trial ahead of schedule and on budget.
Symptoms Of Factor X Deficiency
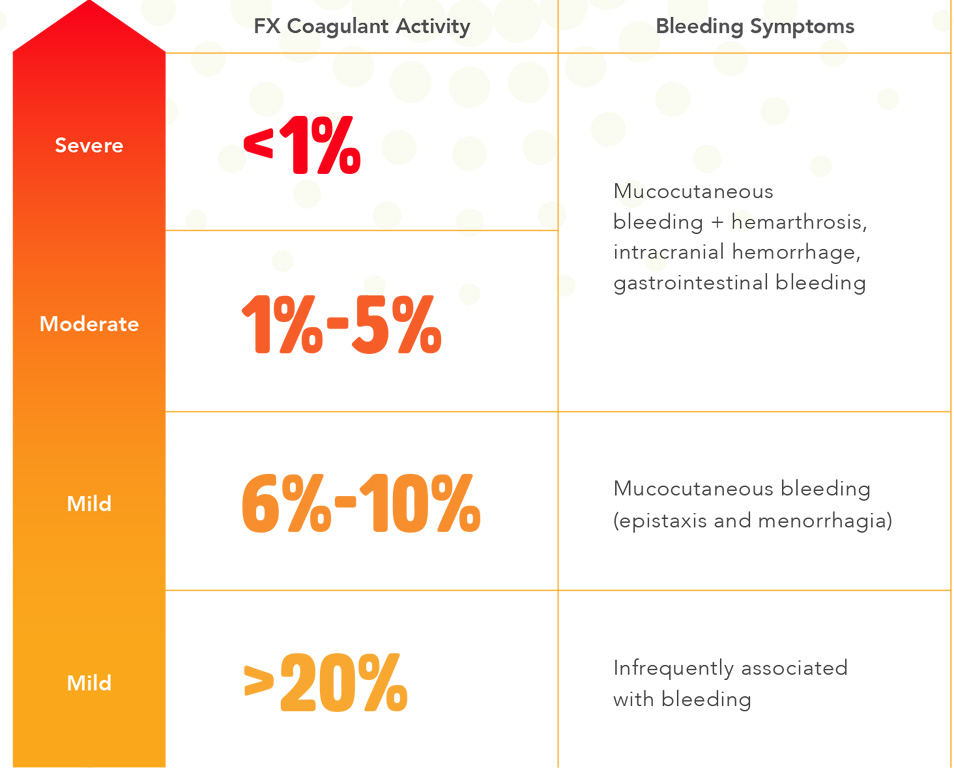
While the signs and symptoms of FX deficiency can begin at any age, the most severe cases typically become apparent in childhood. Severity varies among patients, as do symptoms, with the most common being: nosebleeds, easy bruising, bleeding under the skin, bleeding of the gums, blood in the urine (hematuria), and/or excessive bleeding following surgery or trauma.
Female patients can suffer from menorrhagia or excessive bleeding in childbirth and may also be at increased risk of miscarriage. On occasion, patients may experience bleeding in joint spaces (hemarthrosis).
Patients who are severely affected have an increased risk of intracranial hemorrhage, pulmonary hemorrhage, or bleeding in the gastrointestinal tract, all of which can be life-threatening2.
Treatment Risks
Successful treatment of FX deficiency currently hinges upon the accurate measurement of FX levels. Not providing enough treatment could result in a life-threatening hemorrhage, and too much treatment can cause thrombosis, particularly if intrinsic factor II is high.
Our experience with FX patients has taught us the importance of working alongside participating sites and their staff on topics and/ or techniques to measure FX levels accurately, and making sure that site staff are keenly aware of the many adverse experiences patients may encounter.
We go even further to make sure site staff not only are aware of the potential for an adverse experience but also are able to identify the subtle signs or symptoms of adverse experiences that may occur less frequently or that may be more life-threatening, such as intracranial hemorrhage.
Success In Clinical Trial Recruitment
FX deficiency is relatively rare with an incidence of somewhere between one in 500,000 and one in 1,000,000 patients3. The scarcity of patients makes it even more critical for sponsors to identify the right sites, educate the sites you have, and make sure sites keep you top of mind when potential study patients are encountered.
Choosing Sites
ARG is well known to investigators in the bleeding community. That means that ARG can quickly identify sites known to be treating FX patients, and that personnel at those sites are more willing to consider a potential study when approached by a known CRO with an established working relationship. Finding the right sites, and doing so quickly, means timelines to “first patient in” are abbreviated and overall study timelines can be shortened.
Uniting with Sites
ARG makes open communication a high priority, not just between and among study-specific project teams, but also with treating hematologists. We understand the severity of this condition and recognize breakthroughs when we encounter them. We are also diligent about keeping treating hematologists in the loop, especially in the surgical theater, to share insights and new developments, potentially advise on bleed control, and provide observations for clinical notation.
Additionally, communicating with a site regularly is an important part of the education process, as some site staff may not understand that FX-deficient patients should not automatically be written off as candidates for studies involving surgical procedures. However, there is a fine line between communicating regularly in a helpful manner and communicating too often or ineffectively; those touchpoints or contacts can begin to be perceived as a nuisance.
Being Remembered
ARG is well aware of the importance of making each sponsor’s study stand out in the collective consciousness of investigators and site staff. Our experienced team of clinical support staff are well versed in the fine art of establishing and maintaining a good working rapport with each site.
If a site or sites have a bad experience with a CRO, that experience will likely influence the opinions of site staff toward a sponsor’s study being executed by an unfavorable CRO. When a potential subject visits a site, you want that site’s staff to think of your study first. This is not easy when sites are expected to enroll one or two subjects per year.
ARG Solutions: Reducing Study Start-up Times
ARG uses a variety of techniques to reduce study startup times for FX studies. First and foremost, we rely upon our reputation among sites within the bleeding disorders community.
Simply put, sites want to work with ARG, and that makes identifying sites that are both qualified and interested in study participation a much more efficient process. Sites that trust and enjoy working with a CRO are much quicker to respond to initial inquiries about interest and availability. The contract negotiation process progresses more quickly, too, as familiarity from previous studies can be leveraged.
Leveraging The Hemophilia Treatment Center (HTC) Model
Understanding and then utilizing HTCs to enroll subjects is critical to the timely and successful execution of most, if not al l, FX studies. As a result, it is very important t hat you and your CRO understand how to work with these sites to achieve maximum result.
Key factors to understand include, but may not be limited to:
Structure of the Site
Many HTCs are a part of academic institutions and are staffed by the same people seeing Hematology/Oncology patients: Because they are affiliated with an institution, there is a good chance that the use of a local institutional review board (IRB) will be required.
Knowing how to work with local IRBs is important, but familiarity and experience with a specific site’s local IRB (i.e., awareness of their review schedule, understanding of an institution’s preferred agreement language, familiarity with their documentation requirements, etc.) can help expedite the review and approval process.
There may be competition from competing oncology studies being conducted at the same site. It may be necessary to work with site staff to help prioritize studies so they understand which studies would be of most benefit to a given patient.
Revenue Sources
As with most other prospective research sites, HTCs are businesses. Like most businesses, HTCs must be able to turn a profit. HTCs often use 340B Federal Government Drug Buying Program(s) wherein they purchase products at an established discounted rate, and then sell that product for use on their patients. Clinical studies that use an approved drug as a control could be more attractive to HTCs.
Conversely, a trial that involves the study of a factor replacement product would likely require that a patient use only the research product, which means the HTC will lose the revenue ordinarily generated by selling the patient their replacement factor if that patient had been a participant in the 340B program.
Sponsors need to know how to factor in this economic reality to help make the HTC whole. ARG has helped sponsors work successfully with HTCs by establishing reasonable site payments with some profit built in and providing indirect support through sponsorship of bleeding camps and educational events for HTC staff.
Dispersing Margins Gained
HTCs that use the 340B Federal Government Drug Buying Program(s) must be extremely careful. The difference between the price the HTC pays for products and the price at which products are sold is called the margin.
The use of that revenue generated by those margins is regulated and must be spent to further the comprehensive care model of the HTC or, in some cases, on staff or educational activities.
ARG Solutions: Resourceful Thinking
ARG has come up with some unique approaches to populating trials of surgical patients. For example, we suggest that the sponsor and the FDA modify a prospective study design so that not only could sites that would be performing a specific surgery on future patients be used, but also sites that were known to have performed a specific surgery during the last 12 months could be used as well.
This reliance on retrospective data may reduce the rush to open sites. (If, for example, a surgery is scheduled in one month, but the site takes two months to open, the surgery can be performed as scheduled.
And once the site is open, that procedure and patient data can be collected retrospectively.) It also may allow the sponsor to complete the study with fewer sites than originally projected since they could potentially backfill the trial with centers known to have already conducted the specific surgery on one or more FX-deficient patients.
Moving Forward
Partnering with an experienced CRO that has a full understanding of the intricacies of FX deficiency can be vital to setting up your clinical trial for success.
If you would like to learn more about ARG’s work with various bleeding disorders, other rare diseases and/or overall clinical research experience, contact us at info@atlanticresearchgroup.com.
Refrences
- Brown DL and Kouides PA. (2008). Diagnosis and treatment of inherited factor X deficiency. Haemophilia. 14(6):1176-82.
- Genetics Home Reference: Factor X Deficiency. Retrieved from https://medlineplus.gov/genetics/condition/factor-x-deficiency/.
- National Hemophilia Foundation: Factor X (Stuart-Prower Factor) Deficiency.



